Synthesis of Diarylacetylenes With Triazolium Salts for Antimicrobial Studies — 46a — Liz Dierks, Xavier Gabel
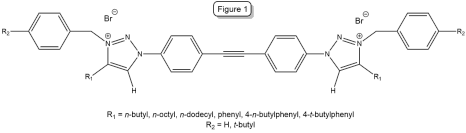
A series of symmetric diarylacetylenes with triazolium salts were created in order to determine their efficacy in killing microbes. It is hypothesized that the rigid diarylacetylene core of these quaternary ammonium salts (QACs) will contribute to the disruption of the cell membranes of bacteria and yeast. Bromophenyltriazoles were synthesized by copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions, which were then converted into symmetric di(phenyltriazole)acetylenes using palladium-catalyzed Stille coupling. Finally, the triazole portion of the acetylene was converted into a triazolium salt (Figure 1) by an SN2 reaction with benzyl bromide derivatives. All reactions afforded good (>60%) yields and all products were characterized by 1H and 13C NMR. Results of minimum inhibitory concentration (MIC) studies against gram-positive bacteria, gram-negative bacteria, and yeast are pending.
Augustana University
Dr. Barrett Eichler
 National Science Foundation RII Track-1 Project:Expanding Research, Education and Innovation in South Dakota
National Science Foundation RII Track-1 Project:Expanding Research, Education and Innovation in South Dakota